Slynd provides effective pregnancy prevention…1,c
(95% CI; 2.3, 6.4)
out of 5547 evaluable cycles
17 out of 953 females evaluated
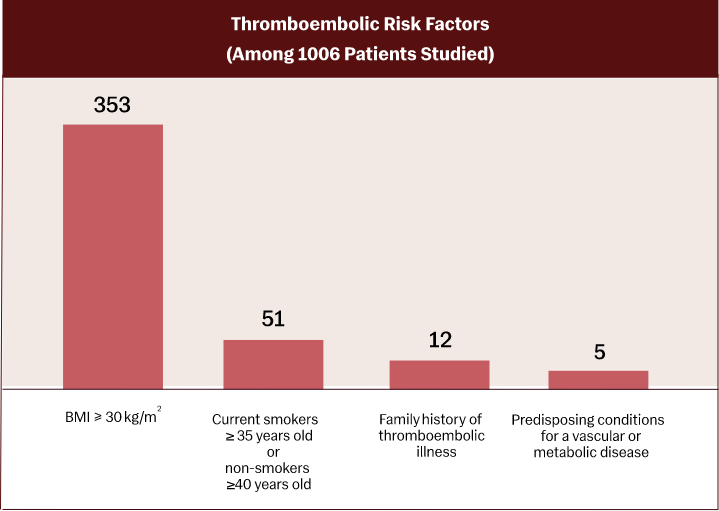
Mean BMI 28.5 kg/m2
332 subjects BMI ≥ 30 (35%)
173 females had BMI ≥ 35 (18%)
c Efficacy was assessed in a single arm trial of 953 women 35 years or younger who were of reproductive potential.
d The data was insufficient to analyze Pearl Index by BMI subgroups.
Slynd has proven in clinical studies with over 3,400 women that it is a safe oral contraceptive² and offers a convenient option for many telemedicine patients.
Slynd does not require a blood pressure check prior to initiation.
No boxed warning!

Unlike estrogen-containing products, there is no evidence of increased risk of myocardial infarction, cerebral thromboembolism, or venous thromboembolism reported in epidemiological studies for progestin-only products.

 Dr. Julie Mullins
Dr. Julie Mullins
A new patient came to me for her annual checkup; she was taking a combination birth control pill with 30mcg of estrogen. Given her smoking and hypertension, I suggested that she switch to Slynd. Now, over a year later, she’s still enjoying her experience with Slynd— highly effective contraceptive, low side effect profile, lighter periodsm. This is just one of many patient success stories I have had with Slynd!
mNot all patients will have the same experience, some patients will experience breakthrough, irregular or no periods.
 Dr. Alan Patterson
Dr. Alan Patterson
Slynd is my number one pill because my patients love it! They appreciate the effects of drospirenone and the adjustment period is just a few months for them to get to a manageable bleeding profile.k And, best of all, I don’t get callbacks!
kIn clinical studies, 3.5% of patients discontinued due to bleeding irregularities.
 Dr. Badeaux
Dr. Badeaux
Our office loves Slynd because it seems to work well in patients over 35 years old who smoke without carrying the estrogen-related riskl of potential increase in blood pressure and blood clots.
IUse of Slynd (drospirenone) in patients with a history of thromboembolic disorders has not been evaluated in clinical trials. Discontinue Slynd if a thromboembolic event occurs.

Find out if Slynd® is a good choice for your patients
References